
Everyday Mysteries Science Inquiry 1 Report
Welcome to Inquiry #1
This is two separate experiments;
A) The way temperature effects a solid ice cube
B) Effects of three different liquids on a solid marshmallow.
Please view the following 8 videos as a time lapse;
GEOS 129 Physical Science for the Elementary Teacher
Everyday Science Mysteries Inquiry 1 Lab Report
Sage Fields, Paige Eichenberger, Elisa Pereyra-Molina, Madison Seamann
Spring 2018
1/31/2018
Experiment 1:
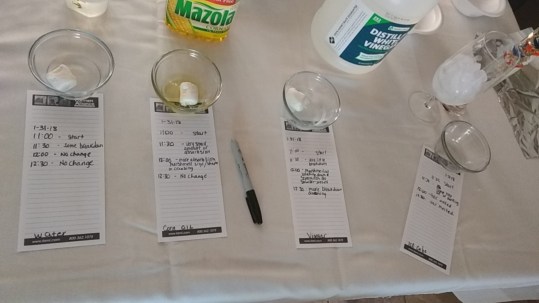
We recorded, in 30-minute increments for 3 hours, the stages a marshmallow dissolving when it sits in 3 different liquids. One marshmallow would be placed in water, another in corn oil, and another in vinegar. As we observe, this experiment will show the effects these 3 liquids have on a marshmallow over time and what happens when a solid begins to dissolve.
Experiment 2:
We recorded, in 30-minute increments for 3 hours, the effects temperature has on a one-ounce cube of ice to demonstrate melting. This experiment was done using the temperature of the current room it was in (72 degrees Fahrenheit). There, we monitored the process of an ice cube naturally melting.
Introduction:
Have you ever seen the marshmallow you plop into your hot chocolate disappear? Or maybe forgot to put away the ice cream you left sitting on the counter and now have to clean up a puddle of liquid? These are everyday examples of objects dissolving or melting. These actions are constantly taking place, whether it is from endothermic or physiological reactions. In this research, we will demonstrate and discuss the effects of objects melting and dissolving.
Research Questions:
Through our research and readings, we were able to determine we wanted to answer two questions in regards to melting and dissolving. Because none of us were comfortable with the topic, we conducted extensive research into vocabulary and constructing a testable hypothesis. Below are the questions that are tested within this report.
- Does a solid (marshmallow) dissolve into water, oil, or vinegar?
- Within this question, we were looking to understand the difference between a chemical change and a physical change as the marshmallow dissolves, creating a new solution.
- How does room temperature affect the melting process of an ice cube?
- By understanding that water freezes at 32 degrees Fahrenheit, we wanted to discover what happens when we turn up the heat. This will help us to understand the effect of temperature on the act of melting from a solid state to a liquid state.
To begin the scientific process, our first goal was to understand the difference between melting and dissolving. We found that melting is the act of changing states, usually with the application of heat. Dissolving is the act of combining two compounds to create one new solution. We had to discuss the aspects of each question, such as time, materials, potential errors, etc. We used web related resources to understand the concept and begin the experiment. Unsure of how this would turn out, our inquiry-based approach helped us to arrive to a conclusion that can be improved and built upon.
Hypothesis:
Experiment #1: Dissolving Marshmallows
- By setting the marshmallow in various types of liquid (water, vinegar, oil), it will slowly absorb the liquid over time and begin to dissolve.
- Prediction: Water will be the most effective in decomposing the marshmallow.
Experiment #2: Temperature Ice Melt
- Because water freezes at 32 degrees Fahrenheit, ice cubes will change states (solid to liquid) at a constant rate at a room temperature of 72 degrees.
Time Constraints: 3 Hour Time Constraint. Data collection will be taken every 30 minutes.
Vocabulary:
While conducting this lab report, there were several terms that had to be researched and defined. Below is a list of words we complied for further analysis.
| Word | Definition |
| Mixture | A combination or blend of different elements or substances. |
| Matter | Something that occupies space |
| Solid | Relatively firm, coherence of particles, or persistence of form as matter |
| Liquid | Composted of molecules that move freely among themselves but don’t separate. |
| Compounds | Composed of two or more parts, elements, or ingredients. |
| Temperature | A measure of warmth or coldness of an object or substance |
| Chemical change | Irreversible chemical reaction involving the rearrangement of atoms of on e or more substances and a change in their chemical properties or composition. Results in the formation of a new substance. |
| Physical Change | Reversible change in the physical property of a substance, as a size or shape. |
| Melting | To become altered from a solid to a liquid state usually by heat. |
| Dissolving | To make a solution of, as by mixing with a liquid, pass into solution. |
| Solutions | A homogenous mixture of two or more substances. |
Procedure and Materials:
Experiment #1: Marshmallows
| Materials | Procedure |
| Jet Puff marshmallows
3 glass bowls Vinegar Oil Water Timer |
1. Collect all necessary materials.
2. Fill one glass bowl with water, one with oil, and one with vinegar. 3. Put one marshmallow in each bowl. 4. After 30 minutes, record data. What do you see? What do you feel? 5. After 3 hours, draw conclusions and determine how to better the experiment. |
Experiment #2: Ice Cube Melt
| Materials | Procedure |
| 1 glass bowl
1 1oz. ice cube Mercury Thermostat timer |
1. Collect all necessary materials.
2. Determine room temperature. 3. Put an ice cube in one glass bowl. 4. After 30 minutes, record data. What do you see? 5. After 3 hours, draw conclusions and determine how to better the experiment. |
Results:
The following link is a video time lapse of the experiment. Within this link, Elisa demonstrated the experiments and made observations every 30 minutes.
CLICK HERE: https://onci2015.wordpress.com/2018/01/31/alpha-science-group-geo-129/
The following graphs were compiled with the data collected from this evidence.
Experiment #1: Marshmallow
| Marshmallow Breakdown | ||||||
| Liquid Type: | Time: | |||||
| 30 Minutes | 60 Minutes | 90 Minutes | 120 Minutes | 150 Minutes | 180 Minutes | |
| Water | Some Breakdown, but still holds shape.
Still firm to the touch. |
Water is slightly foggy, but mostly clear.
No change in shape or touch. |
Absorbed some water. | No change | No change | No change |
| Oil | Small amount of absorption.
No change in color. Still firm to the touch. |
More absorption – size and shape increased.
No change in color. |
No change | Marshmallow is shiny
Size remains the same. |
Absorbed more oil – size is bigger.
Stilly looks shiny. |
No change |
| Vinegar | Very little breakdown.
Vinegar is still clear. Still firm to the touch. |
Beginning to dissolve- marshmallow particles are floating in water. Water is foggy. | More breakdown – water is becoming foggier. | More evidence of dissolving.
Bigger marshmallow particles are floating. |
More breakdown – Marshmallow has lost its shape. | More breakdown – Water is no longer clear. |
Experiment #2: Ice Melt
| Ice Cube Melt | |
| Time | Observation |
| 30 minutes | Has began to melt, but still holds the shape of an ice cube. |
| 60 minutes | Almost completely melted. Small amounts of ice remain. |
| 90 minutes | Ice cube is completely melted. Still cold to the touch. |
| 120 minutes | No change |
| 150 minutes | No change |
| 180 minutes | Melted water is at room temperature. |
After conducting the marshmallow experiment, we can conclude that vinegar is the best liquid to use to dissolve this sweet treat. In the water, the marshmallow began to break down, but at a very slow rate of time. In the oil, the marshmallow began to absorb the liquid and appeared glossy, but there were no signs of dissolving. If the experiment had been extended, we believe the marshmallow would have eventually dissolved completely in the vinegar and the water.
After conducting the ice cube experiment, we can conclude that heat does play an important role in changing a solid to a liquid. Over time, the ice cube melted, and went from 32 degrees Fahrenheit to room temperature.
Pictures:
Discussion:
These experiments would be fun to do in an elementary classroom. By leading with questions, the students will be able to use dialogue and deductive reasoning to come to their conclusion. Though the experiment could use some changes, science is about the learning process. Sometimes we have to adapt and overcome failures. The students would be able to learn from their first lab, revise their work, conduct new hypotheses, and test their work again. This would be an ongoing process until the students found their results satisfying. The fun thing about science is that there is never a wrong question to test, just a better one. By the end of this process, we learned several things about ourselves, our experiment, and the scientific process. Science isn’t easy, but if done right, it is both intriguing and engaging.
Next time, there are several things we could do differently. Some of these would be:
Submerge marshmallow in liquid completely.
Temperature change – see the effect of temperature on ice with more heat (boiling).
Time change – extend time on the marshmallows to see if they dissolve overnight.
Smaller marshmallows – see the effect the liquid would have on smaller marshmallows.
Conclusion:
Our groups experiments focused on the dissolving and melting of a solid into a liquid. The research questions we asked before performing the experiments were: How does room temperature affect the melting process of an ice cube? Does a solid (marshmallow) dissolve into water, oil, or vinegar? Our hypothesis for experiment #1, after setting the marshmallow in various liquids (water, oil, vinegar), it will slowly absorb the liquid over time and begin to dissolve. For our hypothesis for experiment #2, we decided that because water freezes at 32 degrees Fahrenheit, the ice cube would change its state from a solid to a liquid at a constant rate while sitting at room temperature. To see if our hypothesis would turn out correct, we observed every 30 minutes for 3 hours to find out what stage a solid would melt or dissolve at.
In experiment #1 (marshmallows), we had 3 liquids that different marshmallows would sit in. Within the hour we observed that the water and vinegar liquids began to turn a “foggy” color with particles from the marshmallow. And during that first hour, the marshmallow sitting in oil started to absorb the liquid surrounding it, increasing the size and shape. Upon hour 2, the marshmallow had no change while sitting in the water. The second marshmallow in oil had a shiny texture about it, while the size and shape remained the same. Marshmallow 3 in vinegar was still showing evidence of being dissolved slowly, with bigger particles floating in the liquid. Finally, during hour 3, there was no change made to the marshmallows in water and oil. While marshmallow 3 continued to breakdown particles, making the liquid no longer clear. Overall, we discovered that not all of the liquids would dissolve the solid marshmallows like we had anticipated. Only vinegar stripped away particles from the marshmallow, making it slowly dissolve the solid over time. The other two liquids seemed to become absorbed by the marshmallow.
Experiment #2 (ice melting) consisted of an ice cube sitting in an empty bowl at room temperature (72 degrees Fahrenheit). We checked at what state the ice cube was in every 30 minutes for 3 hours. The ice cube starts in the state of a solid and after sitting in the bowl for just 30 minutes, the ice cube has already began melting but still has its original ice cube shape. Observing it after another 30 minutes, we can see the ice cube is almost completely gone with small bits of ice remaining. By an hour and a half the ice cube has melted completely and the water remains cold. On hour number 2 there is still no change, even 30 minutes later the water remains cold. Hour 3, the melted ice turned water is now at room temperature. From the experiment, we can see that the ice melted at a constant rate because of the room temperature, making our hypothesis correct.
We will not reject any of our hypothesis for the 2 experiments. We still consider experiment #1 hypothesis to be correct even though only 1 of the 3 liquids dissolved the marshmallow. We may need to get more in depth for the hypothesis, instead. For experiment #2, we were correct on what the outcome would be and the hypothesis seems to fit the experiment well.
Works Cited
Konicek-Moran, R. (2013). Everyday Physical Science Mysteries. Arlington, Virginia: National Science Teachers Associaiton.
(2018). Retrieved from Dictionary: http://www.dictionary.com.
(2018). Retrieved from Merriam Webster: http://www.merriam-webster.com/dictoinary/dictionary.